QSAR of Substituted Morpholines
Abstract
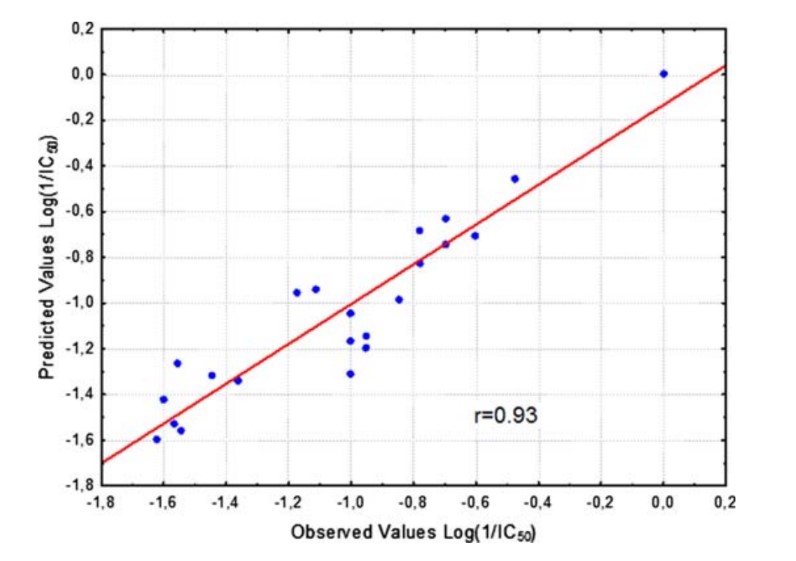
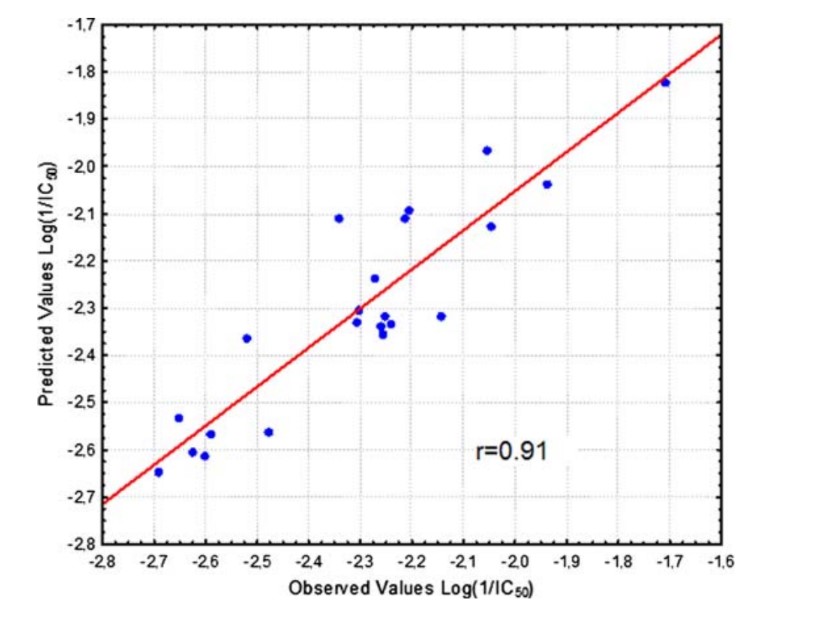
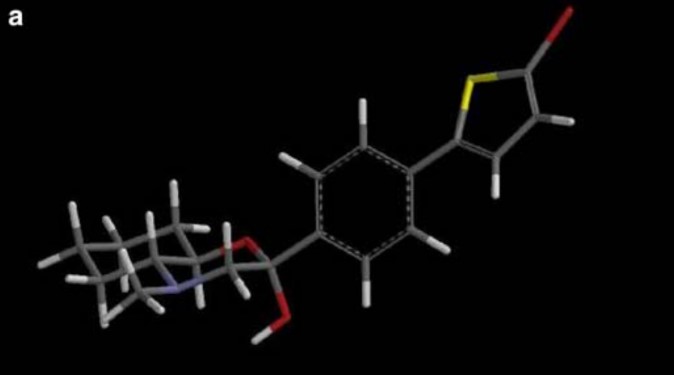
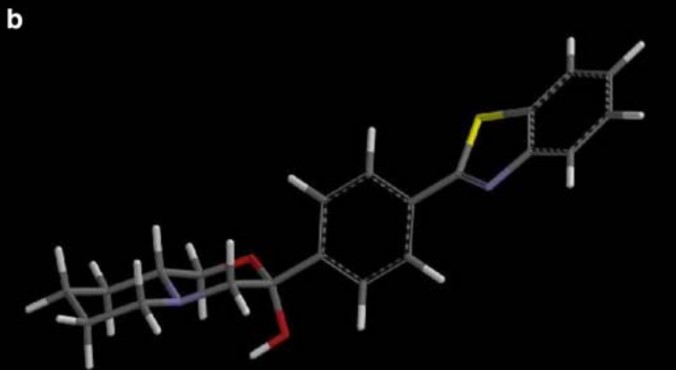
A quantitative structure–activity relationship (QSAR) study was performed for a series of antihyperlipidemic morpholine derivatives, exhibiting squalene synthase (SQS) inhibitory as well as antioxidant activity (inhibition of lipid peroxidation). Physico/stereo-chemical descriptors of low energy conformations of the compounds were calculated and a number of QSAR models with statistical significance and predictability were produced.
The final models include chemical descriptors such as E LUMO, E HOMO–E LUMO gap, cLogP, electrostatic (FNSA1 and RNCS) as well as geometry (YZ SHADOW/YZ RECTANGLE) descriptors, indicating that electron affinity, along with molecular shape and electrostatic effects play a significant role in the compound’s described activities. These models provide some insight on the molecular mechanism of action of these derivatives and assist in the prediction of action in vitro as well as the design of more potent derivatives in the search for effective antiatherosclerosis agents.
Publication Information
- Category: QSAR / Medicinal Chemistry
- Journal: Medicinal Chemistry Research
- Authors: A. Nikitakis, A. P. Kourounakis
- Publication Date: 14 April 2010
- DOI: 10.1007/s00044-010-9351-0
- View Publication